Last Updated on July 27, 2023
Welcome to the world of organic chemistry! In this article, we will delve into the fascinating realm of the Attached Proton Test. This test is a powerful tool used by chemists to analyze the structure and composition of organic compounds. Understanding the theory behind this test is crucial for its successful implementation. We will guide you through the step-by-step process of preparing the sample and setting up the experiment. Additionally, we will explore the interpretation of test results and provide troubleshooting tips for common challenges. Furthermore, we will discuss the applications and importance of the Attached Proton Test in organic chemistry, comparing it with other spectroscopic techniques. So, let’s embark on this journey of discovery and unravel the mysteries of the Attached Proton Test!
Explanation of the Theory behind the Attached Proton Test
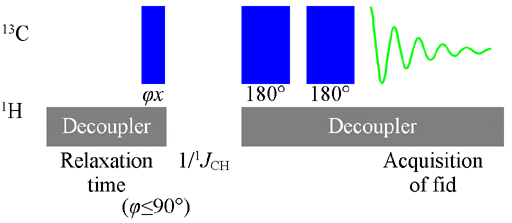
The Attached Proton Test is a spectroscopic technique used in organic chemistry to determine the structure of organic compounds. It is based on the concept of nuclear magnetic resonance (NMR), which involves the interaction of atomic nuclei with a magnetic field.
Here are some key points to understand the theory behind the Attached Proton Test:
- NMR spectroscopy relies on the fact that certain atomic nuclei have a property called spin, which generates a magnetic field.
- When a sample is placed in a strong magnetic field, the atomic nuclei align either with or against the field.
- By applying radiofrequency pulses, the alignment of the atomic nuclei can be manipulated, causing them to absorb and emit energy.
- The energy absorbed and emitted by the atomic nuclei is detected and analyzed to provide information about the chemical environment and connectivity of the atoms in the compound.
- In the Attached Proton Test, the focus is on the hydrogen atoms (protons) attached to carbon atoms in the compound.
Understanding the theory behind the Attached Proton Test is crucial for conducting the test accurately and interpreting the results effectively.
Step-by-Step Guide on Preparing the Sample for the Test
In this section, we will provide a detailed step-by-step guide on how to prepare the sample for the attached proton test. It is crucial to follow these steps carefully to ensure accurate and reliable results.
Step 1: Start by selecting the appropriate sample for the test. The sample should be a pure organic compound that is soluble in the chosen solvent.
Step 2: Weigh the sample accurately using a digital balance. It is important to use the correct amount of sample to obtain reliable results.
Step 3: Transfer the weighed sample into a clean and dry test tube. Make sure to handle the sample with care to avoid contamination.
Step 4: Add the chosen solvent to the test tube containing the sample. The solvent should be selected based on its compatibility with the sample and its ability to dissolve the compound.
Step 5: Mix the sample and solvent thoroughly by shaking the test tube gently. This will ensure proper dissolution of the sample in the solvent.
Step 6: Once the sample is dissolved, transfer a small portion of the solution into a clean NMR tube. The NMR tube should be dry and free from any impurities.
Step 7: Cap the NMR tube tightly to prevent any evaporation or contamination of the sample. The sample is now ready for the attached proton test.
By following these steps, you will be able to prepare the sample effectively for the attached proton test, setting the stage for accurate and reliable results.
Detailed Explanation of the Experimental Setup and Equipment Required
In order to conduct the Attached Proton Test, it is essential to have the right experimental setup and equipment. This section will provide a detailed explanation of the necessary components.
NMR Spectrometer
The most crucial piece of equipment for this test is the Nuclear Magnetic Resonance (NMR) spectrometer. This device is used to measure the magnetic properties of atomic nuclei in a sample. It consists of a powerful magnet, a radiofrequency transmitter, and a receiver.
Sample Tubes
The sample to be tested is placed in a sample tube, which is then inserted into the NMR spectrometer. These tubes are typically made of glass or plastic and come in various sizes to accommodate different sample volumes.
Solvents
In order to dissolve the sample and ensure accurate results, solvents are used. Common solvents include deuterated solvents such as deuterated chloroform (CDCl3) or deuterated dimethyl sulfoxide (DMSO-d6).
Probes
The NMR spectrometer requires a probe to transmit and receive radiofrequency signals. Different probes are used depending on the type of nuclei being studied. For the Attached Proton Test, a proton probe is used.
By ensuring that you have the correct experimental setup and equipment, you can conduct the Attached Proton Test accurately and obtain reliable results.
Procedure for Conducting the Attached Proton Test
Once the sample has been prepared and the experimental setup is ready, it is time to conduct the Attached Proton Test. Follow these steps:
- Start by placing the sample in the NMR spectrometer and ensuring that it is properly aligned.
- Set the parameters for the test, including the temperature, solvent, and pulse sequence.
- Acquire the NMR spectrum by applying the necessary radiofrequency pulses and recording the resulting signals.
- Process the acquired data using specialized software to obtain a clear spectrum.
- Analyze the spectrum to identify the chemical shifts and coupling patterns of the protons in the sample.
- Compare the observed data with known chemical shifts and coupling constants to determine the structure of the compound.
- Repeat the test multiple times to ensure the accuracy and reproducibility of the results.
It is important to note that the Attached Proton Test can be time-consuming and requires a high level of expertise. Therefore, it is recommended to seek guidance from a trained professional or consult relevant literature before conducting the test.
6. Interpretation of the Test Results and Analysis of Spectra
Once the Attached Proton Test has been conducted, it is important to interpret the test results and analyze the spectra obtained. This step is crucial in determining the structure and composition of the organic compound being tested. Here are some key points to consider:
- Examine the chemical shifts: Chemical shifts in the spectra can provide valuable information about the types of protons present in the compound. By comparing the chemical shifts to known values, it is possible to identify the functional groups and determine the structure of the compound.
- Look for coupling patterns: Coupling patterns in the spectra can reveal the connectivity between different protons in the compound. By analyzing the splitting patterns, it is possible to determine the number of neighboring protons and their relative positions.
- Consider the integration values: Integration values in the spectra can provide information about the relative abundance of different types of protons in the compound. This can be useful in determining the ratio of different functional groups present.
- Compare with reference spectra: To confirm the identification of the compound, it is important to compare the obtained spectra with reference spectra of known compounds. This can help in verifying the structure and composition of the compound.
- Use software tools: There are various software tools available that can assist in the interpretation and analysis of spectra. These tools can help in identifying functional groups, predicting chemical shifts, and analyzing coupling patterns.
By carefully interpreting the test results and analyzing the spectra, it is possible to gain valuable insights into the structure and composition of organic compounds using the Attached Proton Test.
Common Challenges and Troubleshooting Tips for the Test
The attached proton test is a powerful tool in organic chemistry, but like any experimental technique, it comes with its own set of challenges. In this section, we will discuss some common issues that researchers may encounter during the test and provide troubleshooting tips to overcome them.
1. Signal Overlap
One common challenge in the attached proton test is signal overlap, where multiple signals from different protons in the molecule appear at the same chemical shift. To overcome this, it is important to carefully analyze the spectra and identify any potential overlapping signals. Adjusting the experimental parameters, such as the pulse sequence or the acquisition time, can help resolve signal overlap.
2. Sample Contamination
Another challenge is sample contamination, which can lead to inaccurate results. To avoid this, it is crucial to handle the sample with clean gloves and use clean glassware for preparation. Additionally, regular cleaning and maintenance of the equipment can prevent contamination.
3. Instrument Calibration
Proper instrument calibration is essential for accurate results. If the instrument is not calibrated correctly, it can lead to distorted spectra. Regular calibration checks should be performed to ensure the instrument is functioning optimally.
By being aware of these common challenges and following the troubleshooting tips provided, researchers can overcome obstacles and obtain reliable results in their attached proton tests.
Applications and Importance of the Attached Proton Test in Organic Chemistry
The Attached Proton Test, also known as the APT, is a crucial technique in the field of organic chemistry. It plays a significant role in the identification and characterization of organic compounds. This test is particularly useful in determining the presence of hydrogen atoms in a molecule, which is essential for understanding its structure and properties.
The APT has a wide range of applications in various areas of organic chemistry. One of its primary uses is in the analysis of complex mixtures, such as natural products or synthesized compounds. By providing information about the number and types of hydrogen atoms present, the APT helps chemists identify and differentiate between different compounds in a mixture.
Furthermore, the APT is invaluable in the study of reaction mechanisms. By analyzing the changes in the proton signals during a chemical reaction, scientists can gain insights into the intermediates and transition states involved. This information is crucial for understanding the underlying processes and designing more efficient and selective reactions.
In addition, the APT is widely used in the field of drug discovery and development. It aids in the structural elucidation of new compounds, allowing researchers to determine their chemical composition and confirm their identity. This information is vital for assessing the potential biological activity and pharmacological properties of the compounds, which is essential in the early stages of drug development.
In conclusion, the Attached Proton Test is a powerful tool in organic chemistry with numerous applications and importance. Its ability to provide valuable information about the presence and arrangement of hydrogen atoms in a molecule makes it an indispensable technique for chemists. Whether it is used in the analysis of complex mixtures, the study of reaction mechanisms, or the discovery of new drugs, the APT continues to contribute significantly to the advancement of organic chemistry.
Comparison of the Attached Proton Test with Other Spectroscopic Techniques
The Attached Proton Test (APT) is a powerful spectroscopic technique used in organic chemistry to determine the structure and composition of organic compounds. However, it is important to understand how APT compares to other spectroscopic techniques in order to fully appreciate its advantages and limitations.
One major difference between APT and other spectroscopic techniques is the type of information they provide. For example, nuclear magnetic resonance (NMR) spectroscopy provides information about the chemical environment and connectivity of atoms in a molecule, while infrared (IR) spectroscopy provides information about the functional groups present in a molecule. APT, on the other hand, provides information about the number and types of attached protons in a molecule.
Another difference is the sensitivity and resolution of the techniques. APT is known for its high sensitivity and resolution, allowing for the detection of even small amounts of attached protons. This makes it particularly useful in the analysis of complex organic compounds. In contrast, other spectroscopic techniques may have lower sensitivity and resolution, limiting their applicability in certain cases.
Furthermore, APT has the advantage of being non-destructive. This means that the sample can be recovered and used for further analysis or experiments. In contrast, some other spectroscopic techniques may require the destruction of the sample in order to obtain the desired information.
In conclusion, while APT is a valuable spectroscopic technique in organic chemistry, it is important to consider its differences and advantages compared to other spectroscopic techniques. By understanding these differences, researchers can choose the most appropriate technique for their specific needs and gain a deeper understanding of the structure and composition of organic compounds.
Wrapping it Up: The Significance of the Attached Proton Test in Organic Chemistry
After delving into the intricacies of the Attached Proton Test (APT), it is clear that this spectroscopic technique holds immense importance in the field of organic chemistry. Through this test, scientists are able to gain valuable insights into the structure and composition of organic compounds, paving the way for groundbreaking discoveries and advancements.
The APT offers a unique advantage over other spectroscopic techniques, as it allows for the identification of hydrogen atoms directly attached to carbon atoms. This information is crucial in determining the connectivity and arrangement of atoms within a molecule, providing a comprehensive understanding of its chemical properties.
By following the step-by-step guide outlined in this article, researchers can confidently prepare samples, conduct the test, and analyze the resulting spectra. However, it is important to acknowledge that challenges may arise during the process. Common troubleshooting tips have been provided to assist scientists in overcoming these obstacles and obtaining accurate results.
In conclusion, the Attached Proton Test is a powerful tool that has revolutionized the field of organic chemistry. Its ability to provide detailed information about the structure and composition of organic compounds has opened up new avenues for research and innovation. As scientists continue to explore the depths of this technique, we can expect even more remarkable discoveries to come.
Learn how to perform an Attached Proton Test in organic chemistry with this step-by-step guide. Understand the theory, setup, and interpretation of results.
About The Author

Zeph Grant is a music fanatic. He loves all types of genres and can often be found discussing the latest album releases with friends. Zeph is also a hardcore content creator, always working on new projects in his spare time. He's an amateur food nerd, and loves knowing all sorts of random facts about food. When it comes to coffee, he's something of an expert - he knows all the best places to get a good cup of joe in town.